Biology Unit 2 Study Guide: Basic Building Blocks of Life
What are biomolecules?
Biomolecules are large molecules made of many smaller repeating units. Therefore they are also considered polymers because they are made of many monomers.
Check out:
BIOLOGY UNIT 3 STUDY GUIDE: CELL ENERGY – PHOTOSYNTHESIS AND CELLULAR RESPIRATION

What helps molecules stick together?
Complete the table about types of chemical bonds.
| Type of Bond |
Formed When… |
|
Covalent Bond |
electrons are shared between atoms
|
|
Ionic Bond |
electrons are transferred from one atom to another and the two atoms are held together by the attraction of opposite charges
|
|
Hydrogen Bond |
attraction between hydrogen atom on one water molecule and oxygen atom on another water molecule – not as strong as covalent or ionic bond
|
Role of Lipids
Lipids have many roles within the cell and body and the function depends on the type of lipid. What are the functions of the following lipids?
| Type of Lipid |
Function(s) |
| Triglycerides |
insulation, cushion organs, stores energy (longer term) |
| Phospholipids |
makes up cell membrane |
| Steroids
(ex. testosterone, cholesterol) |
hormones, cholesterol provides stability in cell membranes |
One physical trait of a lipid that can affect how it behaves is the presence of double bonds between the carbons in the fatty acids. Complete the table below:
|
Type of Bonds Found
(Single/Double) |
Solid or Liquid at
Room Temperature |
Examples |
| Saturated |
single bond |
solid |
butter, animal fats |
| Unsaturated |
contains one or more double bond |
liquid |
peanut oil, vegetable oil |
Role of Carbohydrates
Carbohydrates are best known for their ability to provide organisms with ENERGY. The cells of autotrophs contain chloroplasts (organelle), which has the ability to capture energy from the sun and produce the monomer glucose through the process of photosynthesis – heterotrophs must eat other organisms to get this monomer. BOTH animals and plants break down glucose through the process of cellular respiration in order to get energy in the form of ATP – this is done by the mitochondria (organelle).
Complete the table about carbohydrates.
| Found in … |
Examples |
Monosaccharide or Polysaccharide? |
Function |
| Animals |
glucose |
monosaccharide |
quick energy |
| glycogen (animal starch) |
polysaccharide |
store energy |
| Plants |
starch |
polysaccharide |
store energy |
| cellulose |
polysaccharide |
structure: gives strength and rigidity in cell walls |
Complete the table for testing the presence of carbohydrates.
| Test |
What carbohydrate does this test positive for?
|
What does a positive result look like? |
| Benedict’s Solution |
glucose |
yellow/orange |
| Lugol’s Solution |
starch |
black/dark blue |
Role of Nucleic Acids
The two types of nucleic acids are DNA and RNA. In a eukaryotic cell, DNA is found in the nucleus (organelle). Histones and DNA play an intricate role in gene expression. DNA has the ability to “control” the cell because it codes for the production of the biomolecule protein.
Role of Proteins
Proteins have many, many different functions and in a sense, are really what controls what happens in a cell and even an organism. Complete the following chart on protein functions:
| Type of Protein |
Function |
| Protein channel |
Allows large polar molecules and ions in and out of the cell membrane. |
| Enzyme |
Speeds up rate of reactions to break apart or make molecules |
| Antibodies |
Helps fight diseases. |
| Microtubules and filaments |
Builds the cytoskeleton, gives cell support and allows movement of motor proteins |
| Motor proteins |
Transports materials inside cell along cytoskeleton |
| Hormones |
Allows cells to communicate with other cells. |

Check out:
BIOLOGY UNIT 3 STUDY GUIDE: CELL ENERGY – PHOTOSYNTHESIS AND CELLULAR RESPIRATION
Importance of the Role of Enzymes
Enzymes are critical for allowing biological reactions to occur. Without them, many important reactions (putting together molecules or breaking them apart) simply would not happen.
Describe how the words in the following set are related: (catalyst, enzyme, activation energy, substrate)
A catalyst lowers the activation energy. An enzyme is an example of a catalyst. It works by attaching to the substrate to create a larger molecule or break it apart.
Why are the active site and substrates in an enzyme-catalyzed reaction often compared to a lock and key?
The fit of the substrate to the active site is very precise, therefore they generally only catalyze one reaction.
How do the parts of the cell work together to help the cell function?
A cell can be a living unit all by itself! All the organelles that help a cell function use various biomolecules to perform their jobs. Because it is a living thing, the cell must perform various tasks using organelles just to stay alive. Complete the chart below showing what a cell must do to survive and the role biomolecules play within the organelle.

Function
|
Responsible Cell Part or Organelle
|
| Break down glucose for energy |
mitochondria |
| Digest old materials and get rid of wastes using enzymes |
lysosomes |
| Store materials such as water, lipids, carbohydrates, and proteins for later use |
vacuole |
| Contains DNA which “controls” cell activities by coding for the production of protein |
nucleus |
| Maintain homeostasis by controlling what enters and leaves the cell through the lipid bilayer |
cell membrane / plasma membrane |
| Fills the inside of the cell and allows movement of materials within the cell |
cytoplasm |
| Provides structure within the cell through microtubules and filaments made of protein |
cytoskeleton |
Plant cells have two more organelles that animal cells do not so they can do two more functions, which are:
Function
|
Responsible Cell Part or Organelle
|
| Capture energy from the sun to produce glucose |
chloroplast |
| Provide structure and strength to the outside of a cell through the use of cellulose |
cell wall |
And finally, cells must have a method of building structures and enabling reactions. Ribosomes do this by making PROTEINS.
How do cells regulate their interactions with their environment?
Cells regulate their interactions with the environment through the CELL MEMBRANE, also known as the PLASMA MEMBRANE. Because not everything can pass into or out of the cell, the cell membrane is selectively permeable.
How does the structure of the plasma membrane allow it to be selectively permeable?
Complete the following explanation of the structure of the plasma membrane.
The plasma membrane is composed of a lipid bilayer. Each layer is composed of small molecules called (a) phospholipids. These small molecules have a phosphate head which is polar and a non-polar tail comprised of two (b) fatty acids. The (c) cholesterol molecules dispersed throughout each layer provide stability to the membrane. Other structures called (d) membrane proteins/protein channels are present in the membrane and help substances like glucose enter the cell.
Check out:
BIOLOGY UNIT 3 STUDY GUIDE: CELL ENERGY – PHOTOSYNTHESIS AND CELLULAR RESPIRATION
What is the importance of polarity?
Because fatty acids are non-polar, only non-polar molecules can easily pass through the membrane. Large molecules, molecules with a charge, and polar molecules (except for water), need help from a protein channel.
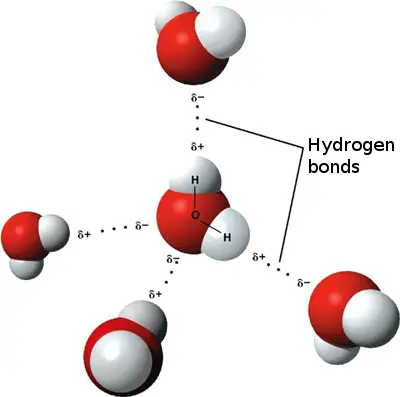
What is polarity and what causes polarity in a water molecule?
Polarity occurs when electrons are not shared equally within a covalent bond. Polarity occurs w/in a water molecule because the electrons spend more time around the oxygen instead of the hydrogens, leaving oxygen with a slightly negative charge and hydrogens with a slightly positive charge.
Other Properties of Water
Complete the table about the different properties of water.
| Water Property |
Definition |
Example |
| Adhesion |
attraction between molecules of different substances |
capillary action – ability of water to “climb” up vessels in plants |
| Cohesion |
attraction between molecules of same substances |
water beads on waterproof material, ability of some insects to walk on water |
| Solvent |
able to dissolve ionic compounds and other polar molecules |
sugar dissolves in water |
Complete the table about acids and bases.
|
Type of ions formed in water |
Location on the pH scale |
| Acid |
H+: hydrogen ions |
below 7 |
| Base |
OH-: hydroxide ions |
above 7 |
How do cells transport substances through the cell membrane?
When molecules move from a high concentration to a low concentration WITHOUT a membrane, this is called simple diffusion. Water can go into or out of the plasma membrane easily. The movement of water molecules across the membrane from high to low concentration is called osmosis. Sometimes larger molecules need the help of a protein channel to move across the membrane. When substances move from high to low WITH the help of a protein, this is called facilitated diffusion. When molecules move across the membrane from a LOW concentration to a HIGH concentration with the help of a protein, this is called active transport. If a cell takes in water or food by wrapping its membrane around it and taking it into the cell, this is known as endocytosis.
Osmosis is the movement of water particles across a selectively permeable membrane from a high concentration to a low concentration. Because water can easily pass through a cell membrane, it’s important for a cell to be surrounded by a solution with the same water concentration. Otherwise, the movement of water can increase or decrease the size of the cell to the point of causing damage.
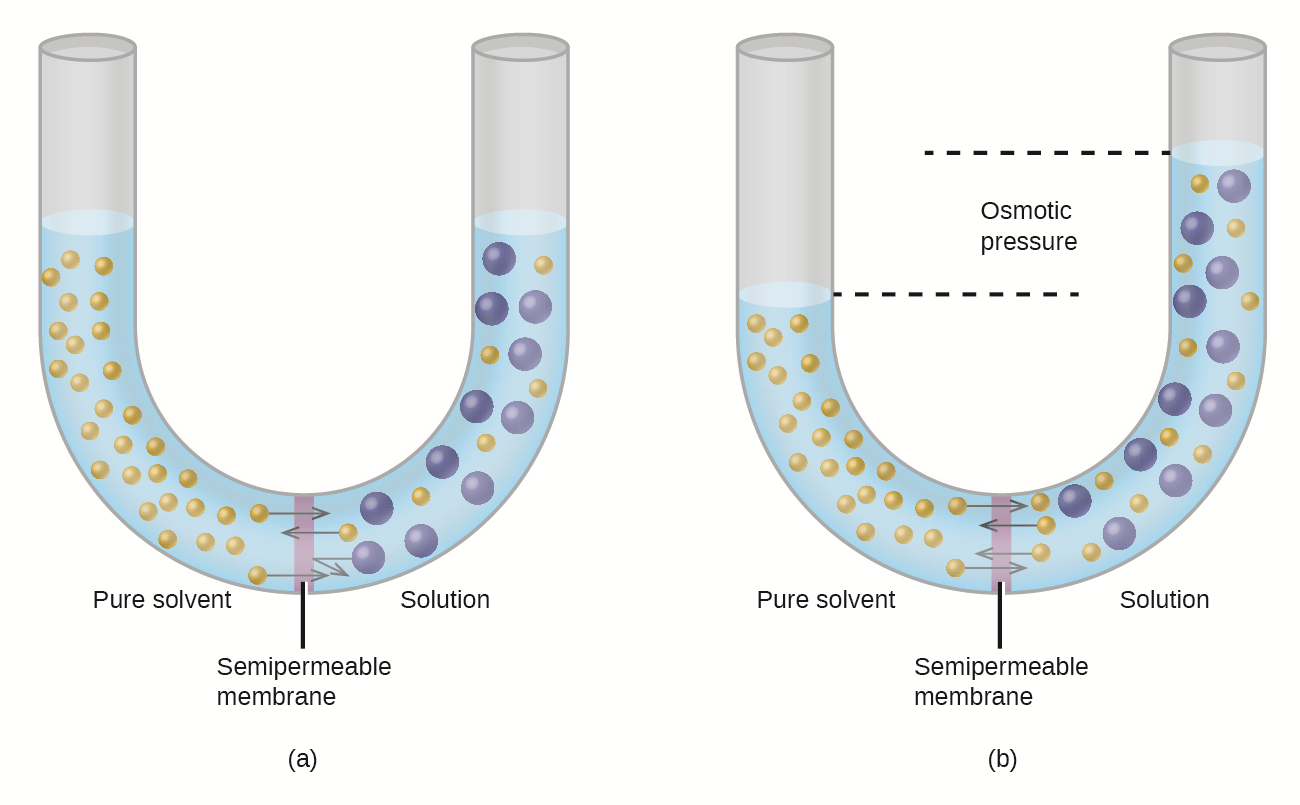
How do you measure the size of a cell?
Practice Problem
- Looking at a cell under high power (25x) the cell diameter takes up 30% of the field of view. If the field of view at low power (5x) is 4.5 mm, what is the diameter of the cell?
4.5 x 1000 = 4,500 mm(micrometers)
4,500 x 5/25 = 900mm (high power f.o.v)
(0.30)x(900mm)=270mm
Thanks for reading, and have a wonderful day!
Check out:
BIOLOGY UNIT 3 STUDY GUIDE: CELL ENERGY – PHOTOSYNTHESIS AND CELLULAR RESPIRATION
Copyright 2022 Moosmosis: All Rights Reserved
Please Like our Facebook page to support our open-access youth education initiatives! 🙂
Works Cited
- FOLCH J, LEES M, SLOANE STANLEY GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497-509.
- Miao KH, Guthmiller KB. Dextran. In: StatPearls. Treasure Island (FL): StatPearls Publishing; May 14, 2020.
- Elias RJ, Kellerby SS, Decker EA. Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr. 2008;48(5):430-441. doi:10.1080/10408390701425615
- Cocinero EJ, Çarçabal P. Carbohydrates. Top Curr Chem. 2015;364:299-333. doi:10.1007/128_2014_596
- Mullegama SV, Alberti MO, Au C, et al. Nucleic Acid Extraction from Human Biological Samples. Methods Mol Biol. 2019;1897:359-383. doi:10.1007/978-1-4939-8935-5_30
Categories: Biology, education, environment, health, medicine, nutrition
Tagged as: answer key, ap biology, Biology, biology study guide, biomolecule, biomolecules, carbohydrate, enzyme, lipid, nucleic acid, organelle, organelles, protein, stem, student, study guide, test, water polarity

















Very informative and well-written!
LikeLiked by 1 person
Excellent biology study guide on the biomolecules.
LikeLiked by 1 person
Helpful, thanks Athena for this great biology study guide! Happy Halloween to everyone!
LikeLiked by 1 person
Thank you dear!! Hope you had a Happy Halloween too!
LikeLiked by 2 people
Reblogged this on nutrition101web.
LikeLiked by 1 person