In this lesson, we explore the nervous system and share notes as part of the study guide series. We will explore the awesome brain and nerves! Topics include Neuron Resting Potential vs Graded Potential vs Action Potential, Axon Capacitance, Action Potential Patterns, and Demyelination Diseases: Guillain-Barre Syndrome vs Multiple Sclerosis .
Check out our popular nervous system notes.

Neuron Resting Potential — Description:
- Positively charged cations are in layer all over outside of the cell membrane; negatively charged anions are in a layer all over the inside of the cell membrane.
- Well, really, there are anions and cations on both sides of the membrane… but more cations on the outside and more anions on the inside.
- Outside is called 0, and difference between outside and inside is usually around -60mV
- Resting potential is related to the concentration differences, or gradients, of different ions across the membrane
- Most important cations that are K+ Na+ and Ca+
- Most important anions are Cl- and organic anions (e.g. proteins)
- Organic anions and K+ have a bigger concentration inside than outside the neuron.
- Na+, Cl-, and Ca2+ have a bigger concentration outside than inside the neuron.
- Each ion is therefore acted on by two forces:
- Electrical potential — ions will be attracted to the side with opposite charge
- ex: OA- electrical force will try to drive it out of the neuron; K+ electrical force will try to drive those ions in (because inside is more negative)
- Diffusion potential — ions will be attracted to the side with lower concentration
- ex: OA- diffusion force has matched diffusion force with electrical force, wanting to drive it out. K+ has opposite diffusion force to electrical force; diffusion force wants to drive it out of cell (even though the inside is more negative).
Neuron Resting Potential — Mechanism:
- Let’s consider a neuron with no resting potential — it’s not more positive outside or more negative inside the membrane, and all the key ions have the same [ ] inside and outside…
- Organic ions are created in the cell for release into the cytoplasm. As this happens, the OA- build up in the cell, creating a small negative membrane potential, but not enough for neuron to function. OA- ions now have electrochemical forces that make it want to leave but it can’t. so there are not further
- For other ions, they can pass through the membrane (unlike OA- ions) through leaky channels that are open all the time.
- The Na+/K+ pump is also in the neuron membrane, and it transports 3 Na+ out and 2 K+ in. This also makes the membrane potential more negative, and increases diffusion gradient/potential for sodium and potassium as more K+ and less Na+ is inside the cell
- The concentration changed inside the cell but not outside because the extracellular fluid is huge, with tons of ions such that any movement into it is negligible.
- For K+, at typical neuron ion concentrations its diffusion force is bigger than smaller electrical force, causing a net movement of K+ out through the leak channels. As they leave, it makes the membrane potential more negative; until equilibrium is reached with K+ ions. This typically occurs around -70 mV which is more than enough for the neuron to function.
- Equilibrium potential / reversible potential = the membrane potential at which there is no net movement of ions across the membrane.
- Doesn’t actually take much for this equilibrium potential to be reached with K+ (maybe 1% of all K+ ions in the cell have to leave), but it does take some time for them to get through leak channels
- For Na+, at typical neuron ion concentrations, Na+ diffusion force and electrical force drive Na+ into the cell. If we had a cell that was only permeable to Na+, it would be driven into the cell to the extent that the membrane potential would switch to positive.
- It would have to be quite positive inside the neuron for the electrical force to balance this diffusion force. Equilibrium potential of sodium is ~ +50 mV.
- Without input, when membrane is at rest, the permeability of the membrane to sodium is much less than permeability to potassium.. It does affect potential a little bit through, so the equilibrium potential is around -60 mV instead of the -70 it would be with just potassium.
- For a cell whose membrane is permeable to multiple ions with electrochemical driving forces, the overall resting membrane potential is a weighted average of those ions’ equilibrium potentials. (weighted by permeability).
- The resting membrane usually has an intermediate permeability to Cl- ions. In contrast to Na+ and K+ whose concentration gradients determine resting membrane potential, the resting membrane potential determines the concentration gradient of Cl- ions. Membrane potential drives Cl- out of the ions until the concentration gradient is big enough to balance it.
- So normally there’s a very small concentration of Cl- inside the cell compared to outside.
- One way chloride is driven out is by the Cl-/K+ symporter, drives Cl- out by harnessing K+ ions’ diffusion force to leave the cell.
- Because of this, equilibrium conc. for chloride is slightly less than resting potential, usually -70mV.. this is usually negligible for overall resting potential, though.
- Ca2+ is also driven out of the cell so that there’s a small concentration of Ca2+ ions inside compared to outside. One way this is down is by the Ca2+/Na2+ exchanger, which harnesses the electrical and diffusion forces acting on Na+ (to bring Na+ in the cell) in order to pump Ca2+ out of the cell.
- This creates strong electrical and chemical gradients for Ca2+ that want to drive it into the cell.
- Equilibrium force of Ca2+ is ~ +120mV, very high, but permeability is very low, so it doesn’t really have an effect on resting potential.
- A neuron at rest (or resting potential) has a stable separation of charges across the membrane.
- At resting potential, there are more positive charges on the layer directly outside of the membrane, and more negative charges on the inside of the membrane.
- This separation of charges refers to polarization. The membrane potential of a neuron at rest is slightly negative, thus it is polarized.
Neuron Graded Potential — description:
- Graded potentials occur in response to input.
- Resting neurons have a stable charge separation across entire membrane, where a layer of cations is on the outside of the membrane (0) and a layer of anions is on the inside of the membrane (~ -60 mV)
- These potentials can be graphed as time vs. potential.
- Inputs from certain types of stimuli may increase or decrease the membrane potential for a brief period of time before it goes back to the resting potential — these are graded potentials
- Tend to occur in the dendrites and soma of the neuron
- Size and duration of graded potential is determined by size and duration of the inputs
- Most graded potentials don’t pass into the axons of neurons but instead most axons have a different membrane potential change called the axon potential.
- Axon potentials start at the trigger zone and occur when the combined effect (summation) of the graded potentials brings the membrane potential of this trigger zone (the initial part of the axon) across a certain value called the threshold potential.
- Threshold potential varies between neurons (but a common one is around -50 mV)
- Summation at the trigger zone is how neurons process information from their inputs.
- Most neurons respond to inputs from other neurons in the form of neurotransmitter molecules released at synapses. Neurotransmitters then bind to receptors on other neuron to produce a graded potential called synaptic potentials.
- Depending on the neurotransmitter and receptor, it could be an excitatory or inhibitory input.
- Other neurons (and neuron-like cells) may also generate graded potentials from physical stimuli, such a slight or odorant molecules. These are receptor potentials.
- Depolarization / excitatory potentials = a graded potential that moves the membrane potential closer to zero (less negative). This moves it closer to the threshold, increasing likelihood of response.
- Hyperpolarization / inhibitory potential = graded potential that moves the membrane potential further away from the threshold, or in the more negative direction. Increases charge separation of the membrane, and decreases likelihood of an axon potential starting.
- Graded potentials decay with time and distance, such that their effect is brief and localized.
- The closer the potential is to the trigger zone, the greater likelihood there will be of it inducing an action potential.
- Therefore a synapse that’s closer to the trigger zone will have a greater influence on the behavior of the neuron
- Temporal Summation:
- If two depolarizations happen slightly separated from one another, they wont’ have any effect.
- But if two happen right around the same time, we get an added effect called a temporal summation that could produce a depolarization twice the size.
- Spatial Summation:
- As graded potentials spread from the dendrites that accepted the neurotransmitters across the soma, they also decay.. so by the time the potential reaches the trigger zone it may not have much of an effect.
- If two graded potentials happen sort of far away from each other, they might not have any effect. But if two graded potentials occur close to each other on the membrane, it could cause spatial summation so you get a depolarization twice the size.
- If you have excitatory input and inhibitory input at the same time and sort of the same place, they may cancel each other out
- Synaptic potentials tend to be quite small, < 1mV in size. So neurons require the temporal and spatial summation of 10 synapses or more to reach threshold and have an effect.
- Resting neurons have a stable charge separation across entire membrane, where a layer of cations is on the outside of the membrane (0) and a layer of anions is on the inside of the membrane (~ -60 mV)
- Electrical synapses do not have a gap between the neuron and target cell – the cells are physically connected.
- Chemical synapses have a gap between the neuron and target to facilitate communication.
- electrical synapses have very different mechanisms to relay information from the neuron to the target cell.
Neuron Graded Potential — mechanism:
- Neurotransmitter receptors are found at synapses and are what the neurotransmitters released from other neurons bind to.
- Many neurotransmitter receptors are a type of ligand gated ion channel
- The graded potential produced depends on:
- What kind of ions pass into membrane (some channels allow just one type in, others allow multiple)
- How many channels are opened (depends on amount of neurotransmitter released into synapse and how long it stays in the synapse)
- If a channel opens that is selective for only one type of ion, the membrane permeability for that ion is increased, which causes the membrane potential for that ion to change around that synapse.
- If Na+ or Ca2+ channels cause a depolarization or excitatory potential, because the cations flow into the neuron and bring positive ions into the more negative internal environment of the cell.
- Force of diffusion and electrical difference drives it in.
- Na+ channel is most common type of depolarization channel.
- If Cl– channel, hyperpolarization usually occurs because it flows into the cell and makes it more negative. (This is most common type of inhibitory channel.)
- Force of diffusion ([ ] outside cell much bigger than in) overcomes the electrical force to drive Cl– ions in.
- K+ channels also cause hyperpolarization because its larger diffusion overcomes small electrical force to drive it out of the cell once the channel if opened, which again makes the internal membrane more negative
- If Na+ or Ca2+ channels cause a depolarization or excitatory potential, because the cations flow into the neuron and bring positive ions into the more negative internal environment of the cell.
- When a neurotransmitter, such as Na+, binds to the receptor and allows, for example, Na+ inside, there will be a small cluster of Na+ ions around that channel that causes membrane potential there to increase.
- It doesn’t just continue increasing, though, because the neurotransmitter leaves and the ion channel closes again. This causes the graded potential to plateau.
- Why do they decay? The Na+ ions diffuse throughout the cell (because of electrical and chemical diffusion) and the depolarization decreases. Eventually that piece of the membrane goes back to its resting potential.
- The sodium-potassium pump pulls potassium ions in and moves sodium ions out of the cell.
- Because potassium is positively charged and the inside of the cell is negatively charged, the electrical gradient tends to pull potassium in, not out.
- When a membrane is at rest, sodium ions or more concentrated outside of the neuron; potassium ions are more concentrated inside. Concentration gradients move potassium ions out of the cell.
- negative sign on a resting potential signifies a relative difference in charge, not an absolute difference.
- Potassium cation is found in greatest concentration inside a neuron in the resting state
- At the resting potential, negatively charged ions will feel an electrical force pushing them out of the neuron,
Graded potentials cause action potentials.
The size of a graded potential must reach a certain threshold in order to cause an action potential.
The size of the action potential is independent of the size of the graded potential (this is known as the all-or-nothing rule). Amplitude doesn’t change.
The best-fit line of the results most likely has the equation y = c.
Difference between action and graded potential:
All potentials are determined by the flow of charged molecules across the neuron membrane.
The difference between graded potentials and action potentials first and foremost has to do with where they occur.
Action potentials occur in axons, while graded potentials occur in the dendrites and soma.
Neuron Action Potential — description:

- Multiple excitatory / depolarizing potentials are needed with temporal and spatial summation to push the membrane of the trigger zone over its threshold potential. When this happens (often around -50 mV), an action potential well be conducted down the whole axon.
- Axon potentials are unlike graded potentials in that they’re the same size for a given neuron (though total size may vary between neurons), and that they’re unchanged (don’t degrade) as they go down the axon, no matter how long it is.
- Shape of an action potential is fairly characteristic:
- After graded potentials reach threshold potential, action potential begins with a rising phase that depolarizes the membrane so much the charge inside the cell membrane is positive, ~ +40mV
- Small plateau
- Rapid falling phase that goes even more negative than normal resting potential, to ~ -70 mV
- Slowly settles back up to resting potential of ~ -60 mV
- All-or-none property of an action potential — you either get one or you don’t (b/c size doesn’t vary for a particular neuron. Doesn’t matter how far over the threshold a graded potential gets, will cause the same size action potential)
- Duration of action potential is also pretty consistent for any particular neuron.
- (Graded potentials have a wider range of duration depending on the duration of their inputs.)
- Speed at which action potentials are conducted can be very fast (1 – 100 m/s).
- Faster speeds usually happen in larger-diameter and more myelinated axons.
- Saltatory conduction — speed of an action potential down a myelinated axon is not consistent — it is conducted faster at the myelinated segments than through the nodes of Ranvier.
Neuron Action Potential — mechanism:
Neuron Action Potential — mechanism:
- The membranes of axons have leak channels and voltage-gated channels (which open when the membrane potential crosses a certain threshold potential)
- Na+ channels are voltage-gated. So when a summation of graded potentials causes depolarization of the trigger zone membrane past the threshold, Na+ flows in. This causes further depolarization at the trigger zone membrane, which then leads to more Na+ channels opening a little further down, and this effects cascades in a wave down the axon.
- Trigger zone has greatest density of Na+ voltage gated channels, which is why the action potential starts there.
- Membrane potential dramatically rises as it tries to increase membrane potential to Na+ eq. potential (around +50 mV). This is rising phase, and the inside of the cell membrane becomes positive.
- After the membrane potential gets depolarized to a certain extent, the Na+ voltage gated channels close, so the membrane potential never actually reaches +50 mV, usually just to +40 mV.
- After they close, they’re in an inactivated state — ion channels are unable to open for a certain period of time.
- After the rising phase and plateau, an exit of K+ through leak channels
and voltage gated channels causes the falling phase of the membrane
potential and it plummets to an even more negative potential (-70 mV)
than at resting state.- K+ leak channels — some K+ leaves when the membrane is at rest
(without input), but after the membrane potential becomes so
positive, both electrical and diffusion force drive K+ out of the
membrane through leak channels quite quickly.
- Voltage-gated K+ channels — also open after membrane potential
crosses threshold, but do so more slowly than the Na+ ones.
- So first, Na+ rushes in causing rising phase, then K+ voltage
channels open (and exit through leak channels increases), causing the falling phase
- Once the membrane potential inside is negative again, it stops falling further because K+ leak channels drive out K+ much slower then, and because the negative potential causes voltage gated K+ channels to close automatically, though again a bit slower than Na+ voltage channels did at positive potential, so the falling action extends past the resting potential for a bit until it settles back.
- This extension past resting potential is called the after-hyperpolarization, or refractory period (called refractory because it’s difficult or impossible to start a new action potential during this period).
- Refractory period has two parts: absolute and relative
- absolute refractory period — when the voltage gated Na+ channels are first closed and they’re in an inactive state. No matter how strong a graded potential comes in, it won’t trigger another action potential.
- relative refractory period — when the voltage gated Na+ channels are functional again, but membrane potential is hyper-polarized. It would take more excitatory input than normal to cause an action potential.
- relative refractory periods can help us figure out how intense a stimulus is — cells in your retina will send signals faster in bright light than in dim light, because the trigger is stronger.
- An effect of refractory period is that action potentials travel down the axon from the trigger zone, and can’t travel immediately back.
- Refractory period determines the maximum frequency at which a single neuron can send action potentials.
- K+ leak channels — some K+ leaves when the membrane is at rest
- Movement of sodium and potassium ions across the membrane starts at trigger zone and spreads in waves. First wave of depolarization all down the axon, then of hyperpolarization, & eventually settling.
- Resting potentials are not associated with refractory periods. Graded potentials are not associated with refractory periods. Refractory periods (both relative and absolute) are times when a membrane is resistant to starting another action potential.
Effects of Axon Diameter and Myelination:
- Axon with larger diameter offers less resistance to ions moving down the axon (more pathways through the cytoplasmic around other cell structures), and therefore allows action potential to be conducted faster (because speed of action potential is related to speed of ions moving down axon).
- Action potentials move faster in myelinated segments because the capacitance of the membrane is reduced. This decreases the number of ions and the time needed to change the membrane potential in these areas.
- Capacitance (in this context) = total number of charges along the membrane, or number of ions that can be stored in the layers on both sides of the membrane at any given potential (because potential represents strength of the charge separation for any particular ion carrier).
- The closer charges are to each other, the more charge can be stored.
- At the nodes of Ranvier, an anion on the inside layer is strongly attracted to cation on opposite side; It overcomes the repulsion of nearby like charges on either side of the membrane and thus in these nodes (at resting potential) more cations/anions can be packed in on either side. (low capacitance)
- In the myelinated segments, the membrane is essentially much thicker, so distance is much greater between oppositely charged ions on opposite sides of the membrane and strength of that charge is less. In myelinated segments you can thus put fewer cations/anions on either side. (high capacitance)
- This means that as an action potential comes rushing by, it is easier to depolarize the areas that are sheathed, because there are fewer negative ions to counteract.
- As our action potential travels down the membrane, sometimes ions are lost as they cross the membrane and exit the cell. The presence of myelin makes this escape pretty much impossible, and so it also helps to preserve the action potential.
- Myelination also decreases the membrane permeability to ions, so fewer total ions cross the membrane during an action potential. Thus fewer ions than normal need to be pumped out through Na+/K+ pump after the action potential.
- Recall that these pumps require energy, myelination actually increases the efficiency of action potential conduction in terms of the energy needed to maintain these ion concentrations after action potentials.
- Myelinated axons have most of their voltage-gated ion channels at the nodes of Ranvier, so they can regenerate the full size of the action potential and keep it strong all the way down the axon.
- More myelin and larger = faster
- Voltage-gated sodium channels are found in lower concentration towards axon terminals.
- Voltage-gated sodium channels are found in greatest concentration in the trigger zones.
Reduced permeability of potassium leak channels would affect the time to reach maximum repolarization in a neuron. (more K+ would remain inside).
- In myelinated axons, action potentials only form in nodes.
- Nodes that are close together might cause action potentials to slow down.
- Nodes that are far apart might cause action potentials to stop.
- Saltatory conduction refers to the the movement of action potentials from node to node.
- Myelinated axons are axons covered with a myelin sheath.
- Myelin sheaths are broken up by small nodes.
- Saltatory conduction is the conduction of action potentials along myelinated axons.
Action Potential Patterns:
- Some neurons fire no action potentials until there is sufficient excitatory inputs. And then the size / duration of depolarization over threshold is converted into the frequency and duration of a series, aka a train of action potentials
- Used by motor neurons that synapse on skeletal muscles.
- Other neurons fire action potentials at a regular rate without any input. This happens because they have differences in their leak channels and/or voltage-gated ion channels that spontaneously depolarize the membrane to threshold at a regular interval.
- Similar to pacemaker cells in the heart.
- With these types of neurons, excitatory input will cause them to fire action potentials more frequently when excited; and when that goes away they go back to their regular rate of firing. Firing is slowed down during inhibition
- During absence of input, some neurons fire clusters of bursts of action, pause for a bit, and then fire more bursts.
- Excitatory input can increases frequency of these bursts (and maybe increase space between them)
- In the last two systems, where neuron fire regularly or in bursts, at resting potentials is that information passed along to target cells can be fine tuned in either direction. (unlike first case, which must be no action potential or train of action potential).
- The last two systems can also pass along info in a more fine-grained fashion.
- The different temporal patterns of action potentials are then converted to the amounts and temporal patterns of neurotransmitter release at the synapse.
- At the peak of an action potential, there is more sodium inside of the membrane than outside.
- Resting potential is approximately -70mV.
- Action potentials peak around 40mV.
- Sodium equilibrium potentials are around 50mV. Thus, the membrane potential is slightly less positive than the sodium equilibrium potential at the peak of an action potential.
- Dendrites receive an action potential.
- Axons transmit action potentials.
- Potassium leak channels allow potassium to exit a neuron in response to depolarization.
- Reduced permeability of a leak channel to its natural ion means that the rate the ions are able to cross the channel is reduced.
- Reduced permeability of potassium leak channels would affect the time to reach maximum repolarization in a neuron.
- Voltage-gated potassium channels play a central role in action potentials.
- Voltage-gated sodium channels also play a central role in action potentials.
- Voltage-gated calcium channels are central to the release of neurotransmitters into the synaptic cleft.
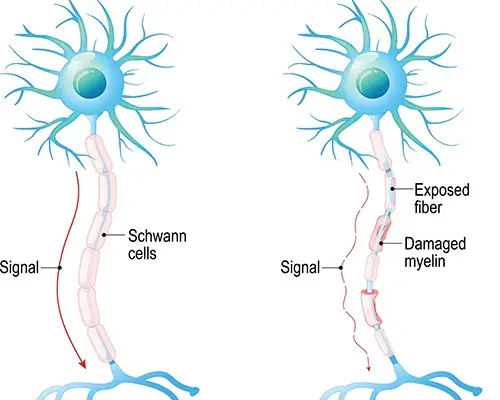
Demyelination Diseases: Guillain-Barre Syndrome vs Multiple Sclerosis
- Demyelination diseases degrade the myelin coating on cells.
- Ex: Guillain-Barre syndrome and Multiple Sclerosis.
- Guillain-Barre syndrome is the destruction of Schwann cells (in the peripheral nervous system)
- MS is caused by a loss of oligodendrocytes (in the brain and spinal column).
- These disorders have different causes and presentations, but both involve muscle weakness and numbness or tingling.
- These symptoms occur because the nerves aren’t sending information the right way. When the myelin coating of nerves degenerates, the signals are either diminished or completely destroyed.
- If the nerves are afferent (sensory) fibers, the destruction of myelin leads to numbness or tingling, because sensations aren’t traveling the way they should.
- When efferent (motor) nerves are demyelinated, this can lead to weakness because the brain is expending a lot of energy but is still unable to actually move the affected limbs.
- Limbs are especially affected, because they have the longest nerves, and the longer the nerve, the more myelin it has that can potentially be destroyed.

Check out our popular nervous system articles!
Central Chemoreceptor vs Peripheral Chemoreceptor
Check out these popular articles 🙂
Circulatory System: Blood Flow Pathway Through the Heart
Ectoderm vs Endoderm vs Mesoderm
Circulatory System: Heart Structures and Functions
Ductus Arteriosus Vs Ductus Venosus Vs Foramen Ovale: Fetal Heart Circulation
Cardiac Arrhythmias: Definition, Types, Symptoms, and Prevention
Upper Vs Lower Respiratory System: Upper vs Lower Respiratory Tract Infections
Seven General Functions of the Respiratory System
Digestive System Anatomy: Diagram, Organs, Structures, and Functions
Kidney Embryology & Development: Easy Lesson
Psychology 101: Crowd Psychology and The Theory of Gustave Le Bon
Introduction to Evolution: Charles Darwin and Alfred Russel Wallace
Copyright © 2022 Moosmosis Organization: All Rights Reserved
All rights reserved. This essay first published on moosmosis.org or any portion thereof may not be reproduced or used in any manner whatsoever
without the express written permission of the publisher at moosmosis.org.

Please Like and Subscribe to our Email List at moosmosis.org, Facebook, Twitter, Youtube to support our open-access youth education initiatives! 🙂
Categories: anatomy, Biology, cell biology, education, health, medicine, stem, technology









![Heart Block: First Degree vs Second Degree (Type I and Type 2) vs Third Degree - ECG Findings, Symptoms, Diagnosis, Treatment, and Prognosis [MCAT, USMLE, Biology, Medicine]](https://moosmosis.files.wordpress.com/2023/04/heart_block.png?w=200&h=200&crop=1)


Brilliantly written, Athena. Thank you for this educational piece! I learned a lot about the nervous system today. 🙂
LikeLiked by 1 person
Thank you so much Moosmosis! Glad you did 😀
LikeLike
Excellent articles
LikeLiked by 1 person
Thank you!
LikeLiked by 1 person
Superb guides on the nervous system!
LikeLiked by 1 person
Thank you K. Stevenson! Superb economics and finance site and articles too!
LikeLiked by 1 person
Thank you! This was really helpful with my studying! Perfecto timing.
LikeLiked by 2 people
So glad to hear! This comment made our day. 🙂 Happy learning!
LikeLiked by 1 person