You can probably find that phosphorus is the 15th chemical element if you look at a periodic table. You might also easily guess that it has the chemical symbol P. But what you may not know is that beyond these basics, there are many different applications of phosphorus that make it a significant part of our everyday lives.

PHOSPHORUS SCIENTIFIC INFORMATION
- Nonmetal belonging to the nitrogen family (Group 15, Period 3)
- Electron configuration: 2.8.5, with 5 valence electrons
- Average atomic weight: 30.9738
- Melting point: 44.1°C / Boiling point: 280°C
- Some physical properties: colorless, waxy, and glow-in-the-dark; solid at room temperature
- One naturally-occurring isotope: P-31
- There main allotropes (physical forms of the element, with different molecular structures): white phosphorus, red phosphorus, and black phosphorus

APPLICATIONS OF PHOSPHORUS
Biological Significance of Phosphorus
Phosphorus is necessary for all known life forms, including the human body, to function.
- Needed for energy production/transportation
ATP (adenosine triphosphate) is used to transport cellular energy in every cellular process that uses energy.
- Grows, maintains, and repairs tissue and cells
Phosphates (compounds containing the phosphate ion, PO4 3−) are the main components of DNA and RNA — the body’s genetic building blocks.

- Maintains the body’s acid-base balance
Phosphorus is one of the body’s main buffers (chemicals that maintain the pH of a system by absorbing hydrogen ions—making it more acidic, or absorbing hydroxyl ions—making it more alkaline). This is crucial in keeping up the normal physiology and metabolism in our bodies.
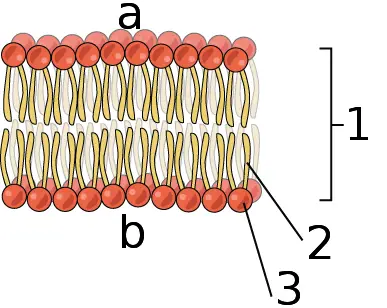
Phosphorus forms the main structure of all cell membranes as phospholipids
Phospholipids are composed of hydrophilic (water-loving) phosphate group heads and hydrophobic (water-hating) fatty acid tails. In water, the phospholipids instantly arrange themselves in a bilayer with phosphate group heads on the outside and fatty acid tails on the inside. Through this structure, they are able to form cell membranes which protect the cell content.
Crucial for bone & teeth growth
Calcium phosphate salts called hydroxyapatite are the main components of bone (50% by volume, 70% by weight) & tooth enamel in the human body.
Due to this extensive use of phosphorus in our bodies, when we lack phosphorus levels, there are adverse consequences to our health. Some effects of phosphorus deficiency include bone pain, stiff joints, fatigue, irregular breathing, irritability, numbness, and muscle weakness.

Medical Uses of Phosphorus
Phosphorus Lab Tests
Phosphorus lab tests are used to evaluate the level of phosphorus in the blood to aid in medical diagnosis. They are performed after an abnormal calcium test, or when problems like fatigue, muscle weakness, cramping, or bone problems have been detected. They can also be used to test for kidney and gastrointestinal disorders, and phosphorus levels may be monitored for diabetes patients or someone who has an acid-base imbalance.
Phosphorus supplements
Phosphorus supplements can be used by athletes before matches and heavy exercise workouts with the goal of reducing muscle pain and fatigue. They are also prescribed to patients who can’t get enough phosphorus in their diet due to an illness: for example, elderly osteoporosis (bone weakness) patients take calcium supplements. This high dose of calcium citrate/calcium carbonate can bind up to 500mg of phosphorus in the body, making it unavailable to the body, causing phosphorus deficiency. Phosphorus supplements can fix that.

Bone grafting materials and dental prosthetics/repair
Hydroxyapatite (calcium phosphate salt) is used as an ingredient in these instruments, which are extremely beneficial for patients of diseases, traumatic events, or congenital conditions. This implant helps to sustain an interlocked, porous structure, helping cellular development & tissue regeneration. This also promotes rigid attachment between the implant and the surrounding tissue, blocking the growth of other fibrous tissue and restoring the function.
However, it must be noted that pure HA is relatively brittle and unsuited for supporting weight. Hence, it’s not used in its pure form, and is instead used as composites with other materials.
Industrial/Commercial Uses of Phosphorus
Phosphorus plays an important role in various industries: for example, the production of steel, fine glasses & chinaware, or as ingredients in soft drinks, pesticides, and fireworks.
Fireworks/pyrotechnic devices
The heat produced when lighting a firework converts red phosphorus to white phosphorus, which then ignites & combusts when exposed to air.
White vs Red Phosphorus
White phosphorus is used in flares & incendiary (flammable) devices, because it’s highly reactive and can thus spontaneously ignite when in contact with air. This helps to ignite and sustain the burning of flares, which can be used as signals or for illumination.

Red phosphorus is an ingredient used in the strip of material on the side of matchboxes, so when we light matches, we strike the matchstick against this surface containing red phosphorus.
This striking strip on modern matchboxes is usually composed of 25% powdered glass, 50% red phosphorus, and 16% binder. The head of the matchstick is made of 50% potassium chlorate, along with sulfur and starch.
When the match is struck, the phosphorus and chlorate mix, which ignites due to the friction. This heat breaks down potassium chlorate, releasing oxygen in the process. This oxygen allows the flame on the match to sustain itself.
Fertilizers
Phosphates and phosphorus compounds are used in fertilizers, and they are critical to plants due to their various functions mentioned above in the “Health” section. Many of the biochemical processes that are essential for plant metabolism, like photosynthesis and energy production, require these phosphorus-containing substances to be successful.
Soft Drinks
Phosphoric acid is added to soft drinks to improve shelf life. It prevents the growth of molds and bacteria in sugary drinks, such as in cola or soda. It also gives carbonated drinks a sharper, crisper aftertaste, making the product more appealing to consumers.
In summary, phosphorus is extremely valuable to our health because it plays a crucial role in the human body due to its many functions, and also has many medical applications. Furthermore, its use in manufacturing many products including steel, fertilizer, soft drinks, and fireworks proves that it’s a significant benefit to our industries.

Works Cited
“Phosphorus.” Encyclopædia Britannica, 9 May 2018, http://www.britannica.com/science/phosphorus-chemical-element.
“Phosphorus.” Lab Tests Online, AACC, 23 July 2018, labtestsonline.org/tests/phosphorus.
D., Cabrol, et al. “Phosphorus Element Facts.” Chemicool, http://www.chemicool.com/elements/phosphorus.html.
“Phosphorus – Element Information, Properties and Uses.” Royal Society of Chemistry, Royal Society of Chemistry, http://www.rsc.org/periodic-table/element/15/phosphorus.
“Phospholipid.” Biology Dictionary, Biology Dictionary, 29 Apr. 2017, biologydictionary.net/phospholipid/.
“Glossary.” Linus Pauling Institute, Oregon State University, 10 Aug. 2018, lpi.oregonstate.edu/mic/glossary#buffer.
“Bones Need Both Calcium and Phosphorus.” WebMD, 20 Mar. 2002, http://www.webmd.com/osteoporosis/news/20020320/bones-need-both-calcium-phosphorus.
Habibah, Tutut Ummul. “Dental Materials, Hydroxyapatite.” NCBI, 16 Sept. 2018, http://www.ncbi.nlm.nih.gov/books/NBK513314/.
“Phosphorus.” Wikipedia, Wikimedia Foundation, 10 Sept. 2018, en.wikipedia.org/wiki/Phosphorus.
Dubois, Sirah. “Is Phosphorus in Soda Bad for You?” The Nest, 14 July 2016, woman.thenest.com/phosphorus-soda-bad-you-3328.html.
© Copyright 2020 Moosmosis – All rights reserved
All rights reserved. This essay or any portion thereof
may not be reproduced or used in any manner whatsoever
without the express written permission of the publisher.














Great facts!
LikeLiked by 1 person
Very interesting article on phosphorus!
LikeLiked by 1 person
Highly useful for our children to learn. Thanks. Keep posting.. Kudos
LikeLike