What are the factors that determine whether a molecule can cross a cell membrane?
There are 3 important factors that determine whether a molecule can move or cross through a cell membrane: 1) Molecular Size, 2) Concentration, and 3) Molecular Charge or Polarity.
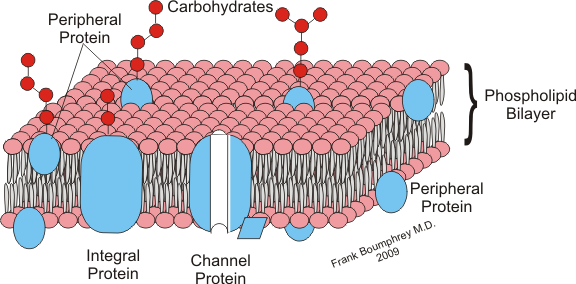
1. Molecular Size
The larger the molecule is, the harder it is to cross through the cell membrane.
The smaller the molecule is, the easier it is to cross through the cell membrane.
2. Concentration
Molecules like spaces that are less crowded, so when one side of the cell membrane has a low concentration of that same type of molecule, the molecules can cross the cell membrane more easily. For example, when there is a higher concentration of oxygen outside the cell and a lower concentration of oxygen inside the cell, oxygen molecules diffuse better as they enter the cell, or the low oxygen concentration side. This is how our red blood cells, low on oxygen, can pick up more oxygen in the highly oxygen dense lungs.
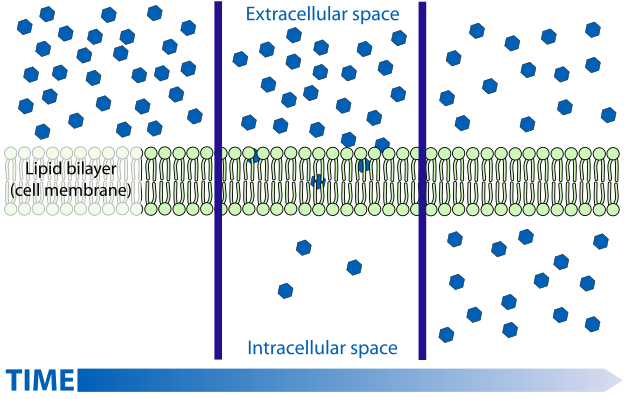
3. Molecule Charge or Polarity
The more polar the molecule is, the harder it is to cross through the cell membrane.
The less polar or more nonpolar the molecule is, the easier it is to cross through the cell membrane.
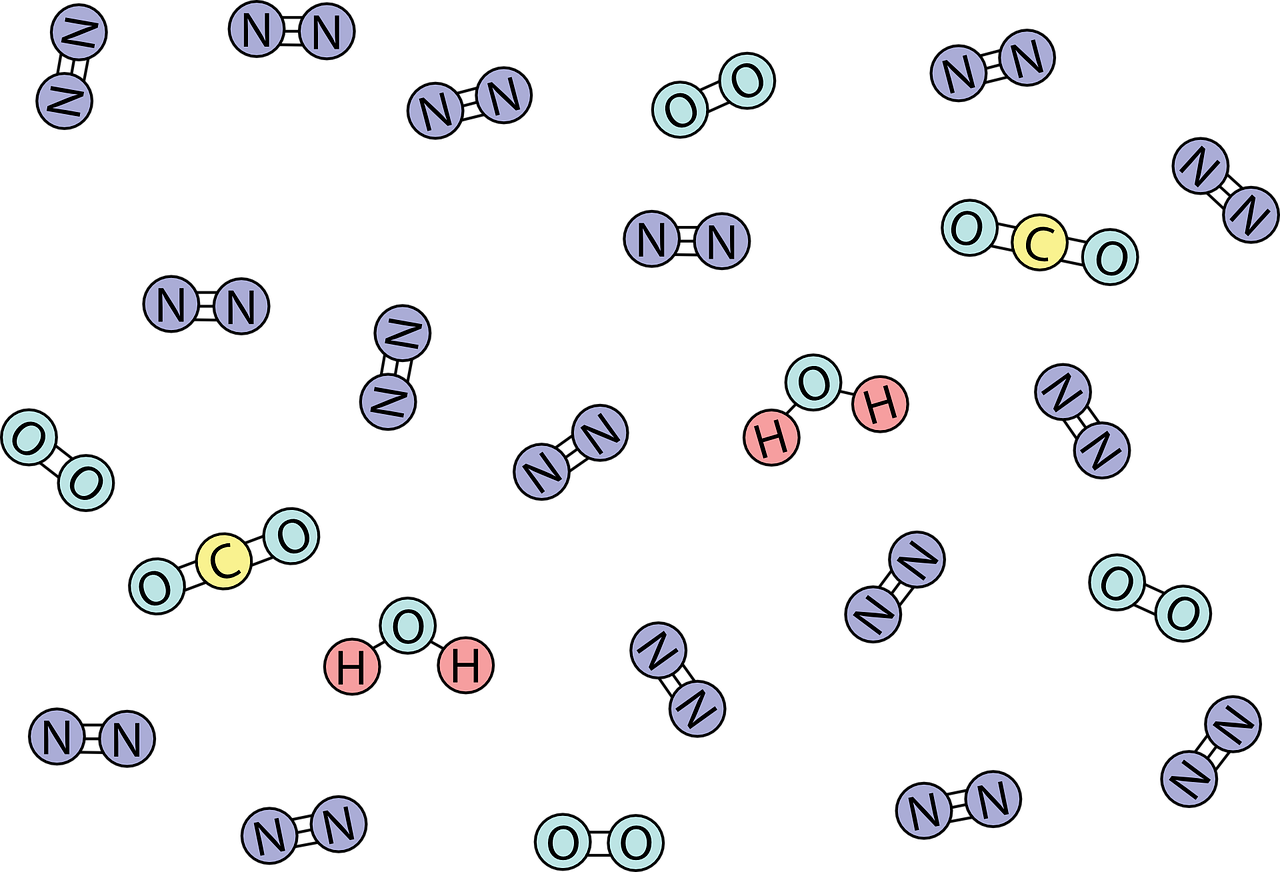
General Order Summary of Molecule Types that can pass through the cell plasma Membrane
All 3 of these aforementioned factors combine together to play a role on whether or not a molecule or ion can cross through the cell membrane, the phospholipid bilayer. In this section, we share a general summary of the types of molecules that can diffuse through the cell membrane in order of difficulty of passing through.
Gases such as Oxygen and Carbon Dioxide (CO2) can pass freely through the cell membrane. Small polar molecules such as water of H2O can pass but very slowly. They are usually assisted through facilitated diffusion such as with osmosis.
Large nonpolar molecules such as benzene are very slow in passing through. The larger the nonpolar molecule, the slower it can pass through the membrane. For example, ethylene is C2H4, which is smaller than the molecular composition of benzene, C6H12. Since ethylene is smaller than benzene, ethylene can pass through the cell membrane faster relative to benzene (albeit both are slow in passing through compared to gases or small polar molecules like water and ethanol).


The larger the nonpolar molecule, the slower it can pass through the membrane.
Large polar molecules cannot pass through diffusion. This includes glucose. Lastly, charged polar molecules cannot pass through. Both large polar and charged polar molecules would require energy or ATP to be transported across the cell membrane. This can occur through active transport.
Question: What types of molecules can diffuse easily through a cell membrane?
Putting everything together, small nonpolar molecules like oxygen and carbon dioxide can diffuse easily through a cell membrane. Many ask, “Can water diffuse easily through a cell membrane?” Water can diffuse through a cell membrane through aquaporin proteins and osmosis, but water cannot diffuse as easily as small nonpolar molecules like oxygen and carbon dioxide.
Question: What types of molecules cannot diffuse easily through a cell membrane?
Larger sized and more polar charged molecules cannot diffuse easily through a cell membrane. Examples of molecules that cannot diffuse easily through a cell membrane include glucose and polar charged molecules like sodium (Na+), potassium (K+), and chloride (Cl-). These molecule types require ATP energy or active transport to pass through the cell membrane.
Question: Can ions cross the Lipid Bilayer by simple diffusion?
No, ions cannot cross by simple diffusion or osmosis. Ions are charged molecules. Even if they are small sized, their charges create polarity which would not allow them to pass through the lipid bilayer easily. Therefore, ions pass through the cell membrane through active transport via protein channels or pumps, or they can cross through the lipid bilayer through facilitated diffusion.
Please note that simple diffusion is not facilitated diffusion and that osmosis refers to the movement of water, not ions.
Here is a simplified table summarizing general molecule types that can pass through the cell plasma membrane in order from easy to Difficult.
[Diffuse Easily] Gases (CO2, O2) > Small Polar (H2O) > Large Nonpolar (Benzene) > Large Polar (Glucose) > Charged Polar Molecules (Cl-, K+) [Harder to Pass through/Needs Active Transport]
Works Cited
- Nature. Cell Membranes. https://www.nature.com/scitable/topicpage/cell-membranes-14052567
- NCBI. The Cell: A Molecular Approach 2nd Edition. https://www.ncbi.nlm.nih.gov/books/NBK9928/
- General Chemistry. 4th Edition. Donald McQuarrie.
© Copyright 2019 Moosmosis – All rights reserved
Answers to Practice Test on “What Types of molecules can pass through the cell membrane?
- Oxygen can pass through the cell membrane easily because of the nature of its small size! Do not get confused by the tricky answer choices. Oxygen passively crosses the cell membrane and does not need an active transporter or energy from ATP.
- *Hint* Red blood cells that just exited out from the lungs are highly oxygenated: high O2 and low Co2. Your body cells (legs) have low O2 and high Co2. Remember, it’s easier for a molecule to diffuse across the cell membrane from a high concentration to a low concentration. Co2 would thus diffuse out from the body cells (high concentration) to the red blood cells (low concentration). This does not need any energy/active transport.
- H20! In comparison to the other molecules, H20 is the most polar and thus by relative comparison, cannot pass as easily through the cell membrane. We have to remember, H20 diffuses through the cell membrane with the help of special cell structures called aquaporins. The other 3 answer choices, N2, O2, and Co2 are all nonpolar gasses that pass through the cell membrane much easier than H20 can.













This is a good article for me. Very interesting to me.
LikeLiked by 1 person
Thank you for learning with us!
LikeLike
This is a good article for me. I study an artificial cell membrane now. I learn a lot from this article.
LikeLiked by 1 person
Thank you so much for your kind comment! We’re glad you enjoyed the article. Happy learning!
LikeLiked by 1 person
Helpful clear thanks!
LikeLiked by 1 person
You’re very welcome, Dan. Glad to help. Happy learning!
LikeLike
Beautifully written and very helpful info on molecules and the cell membrane!
LikeLiked by 2 people
Not accurate. Ions can use facilitated diffusion or active transport. It just depends on the concentration.
LikeLike
In our article, we wrote that ions cannot cross by simple diffusion, which is true. Simple diffusion is not the same thing as facilitated diffusion, and osmosis is a specific term that refers to water transport. It is also true that ions can use facilitated diffusion, as you have mentioned. We thank you for your comment and have updated the article for clarification.
LikeLiked by 1 person
I agree!
LikeLiked by 2 people
that helped me a lot thanks
LikeLiked by 2 people
You’re very welcome! Glad to help, and happy learning! 😀
LikeLiked by 1 person
Yaas! An article that explains it so well ❤️
LikeLike
Great info and very well explained.Thank you!
LikeLiked by 2 people
Thank you for your kind comment! Glad that this helped! 🙂
LikeLiked by 1 person